Objective The aim of this study was to understand the impact of training with a hand robotic device on hand paresis and function in a population of children with hemiparesis. Methods Twelve children with hemiparesis (mean age, 9 [SD, 3.64] years) completed participation in this prospective, experimental, pilot study. Participants ...
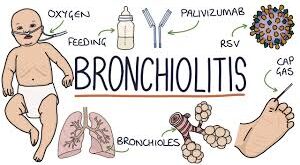
Leer Más »Bronchiolitis: diagnosis and management of bronchiolitis in children
Overview This guideline covers diagnosing and managing bronchiolitis in babies and children. It aims to help healthcare professionals diagnose bronchiolitis and identify if babies and children should be cared for at home or in hospital. It describes treatments and interventions that can be used to help with the symptoms of bronchiolitis. In August ...
Leer Más »Canadian 24-Hour Movement Guidelines for the Early Years (0–4 years): An Integration of Physical Activity, Sedentary Behaviour, and Sleep
Abstract Background: The Canadian Society for Exercise Physiology convened representatives of national organizations, research experts, methodologists, stakeholders, and end-users who followed rigorous and transparent guideline development procedures to create the Canadian 24-Hour Movement Guidelines for the Early Years (0–4 years): An Integration of Physical Activity, Sedentary Behaviour, and Sleep. These ...
Leer Más »Pfizer’s vaccine shows strong response and is safe in children ages 5 to 11
The companies Pfizer and BioNTech announced on Monday the results of a phase 2/3 trial showing a favorable safety profile and robust neutralizing antibody responses in children aged 5 to 11 years also with a two-dose regimen administered with 21 days. difference . The dose was 10ug , a lower dose than the 30 ug dose used for ...
Leer Más »The EMA approves Moderna’s vaccine for young people between 12 and 17 years old
The Committee for Human Medicines (CHMP) of the European Medicines Agency (EMA) has recommended granting an extension for the vaccine against COVID-19 Spikevax (formerly known as COVID- 19 Vaccine Moderna) to include use in young people aged 12-17 years . The vaccine was already authorized in people over 18 years of age. The use of ...
Leer Más » Blog de Fisioterapia Fisioterapia
Blog de Fisioterapia Fisioterapia